
Home
Lost Reg Code?
News
Online KiwiSDRs
macOS Software
Windows Software
Ham Radio iPhone/iPad
Linux Software
Support/FAQ
Update Policy
USB Icom CI-V Interface
Radio Hobbyist Products
22m Programmable Beacon Kit
CW Keyer For Beacons Kit
Jellyfish Transformer
Cyclops Antenna Transformer
General Interest Programs
Atomic Mac/PC
Audiocorder
Audio Toolbox
Black Cat Timer
Diet Sleuth
iUnit
Graffikon
Graph Paper Maker
Health Tracker
Image Resizer
Knitting Wizard
Label Wizard
Prog Audio Gen
Sound Byte
Synth 76477
Amateur Radio Programs
AirSpyHF+ Server
Audiocorder
Black Cat Absolute ACARS
Black Cat ACARS
Black Cat ALE
Black Cat ALE Vacuum Cleaner
Black Cat NetFinder
Black Cat GMDSS
Black Cat HF Fax
Black Cat SSTV
Black Cat NAVTEX SITOR-B
Carrier Sleuth
Cocoa 1090
Cocoa RTL Server
DGPS Decoding
DX Toolbox
Elmer
MatchMaker
KiwiKonnect
KiwiSDR Monitor
KiwiSDR Sound Client
MININEC Pro
Morse Mania
MultiMode
sdrRewind
RF Toolbox
SDRuno Plugin
SDRuno Equalizer Plugin
SelCall
Sonde
iPhone/iPad Apps
ALE
Atoms To Go
dB Calc
Clik Trak
DGPS Decoder
Drill Calc
DX Toolbox
Elmer Extra
Elmer General
Elmer Tech
Feld Hellschreiber
Field Strength Calc
Function Generator Pad
GMDSS
Godafoss
HF Weather Fax
iAttenuate
iFunctionGenerator
iSoundex
iSoundByte
iSweep
iUnit
Morse Mania
ACARS Pad
Morse Pad
NAVTEX Pad
Packet Pad
PSK31 Pad
SSTV Pad
Photon Calc
Rad Map Tracker
RF Link Calc
SelCall Tone Gen
Sound Byte
Sound Byte Control
Spectrum Pad
SWBC Schedules
Synth 76477
Synth Motion
Transmission Line Calc
Weather Calc
Wire Calc
iPhone/iPad Bundles
RF Calculator Apps
Ham Radio Decoder Apps
Audio Utility Apps
Shortwave Weather Apps
Ham Radio Exam Study Apps
Shortwave Decoder Apps
About Black Cat Systems
Site Map
Our software for Mac OSX
Privacy Policy
Press/Media
HFunderground
Apple /// Emulator
Macintosh Links
Shortwave Radio
Pirate Radio
Spy Numbers Stations
Science and Electronics
Ham Radio Software

Radioactive Products and Other Sources Of Radiation
New! See our tests of radiation from the iPad and iPod
Purpose:
On this page, I discuss some of the products that either used to be available, or are still available, that contain radioactive components, and some of the interesting results I've found.Measurements of the radiation levels of these items was taken using the GM-10 and GM-45 radiation detectors
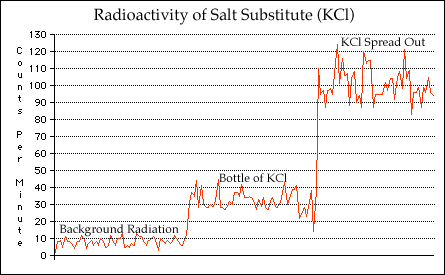
Radioactive Salt Substitute:
Watching your sodium intake by using a salt substitute? Well, while you may be decreasing your sodium intake, you're also slightly increasing your radiation levels. Salt substitutes contain Potassium Chloride, rather than Sodium Chloride (common table salt).As it turns out, approximately 0.01% of the Potassium found in nature is Potassium-40, a radioactive isotope with a half life of 1.28 billion years. It's a beta emitter, with an energy of 1.3 MeV (millions of electron volts). Due to the very long half life, the activity is rather low, meaning you need a bit of it to actually get a measurable quantity of radiation.
As an experiment, I bought a small (3.5 ounces, 100 grams) bottle of it from the supermarket
and placed it in front of my geiger tube (pancake style with a mica window). The reading
jumped from around 8 counts per minute (CPM) to 35 CPM, more than a factor of four. I then
poured a small amount (perhaps a tablespoon) onto a piece of paper, and then positioned the
GM detector just above the salt. The reading went up to around 100 CPM. While not a huge
amount of radiation, it is about 12 times the background level, and quite detectable.
Smoke Detectors:
Smoke detectors contain a small amount (1 microcurie) of Americium-241, a radioactive element. It has a half life of 460 years, and emits alpha particles (around 5.5 MeV) and low energy gamma radiation (mostly at 60 keV). A voltage applied to two plates forms an ionization chamber inside the detector. The Americium is used to ionize the air between the plates, causing a current to flow. Smoke entering the detector blocks some of the alpha particles, lowering the current, and triggering the alarm.
As a test I first checked the outside of the detector with a GM tube. There was no apparent increase
over background, which is to be expected, since the alpha particles
are stopped inside the detector.
Opening the detector and exposing the Americium source, I was able to get a substantial reading, which
increased as I moved the GM tube close to the source. It finally reached around 19,000 CPM with the
GM tube almost touching the source. Again, I should stress that outside the detector housing, there is
no increase in radiation over normal background levels.
Radioactive Spark Plugs
 Yes, it's true... back in the 40's, some types of spark plugs had trace
amounts of polonium in the gap, presumably to ionize the air. This allowed
a larger gap to be used. I'm not a motor-head, but I guess a larger spark
gap is a good thing, presumably because it would result in a higher
voltage before breakdown.
Yes, it's true... back in the 40's, some types of spark plugs had trace
amounts of polonium in the gap, presumably to ionize the air. This allowed
a larger gap to be used. I'm not a motor-head, but I guess a larger spark
gap is a good thing, presumably because it would result in a higher
voltage before breakdown.
Fiestaware

The Homer Laughlin China Co. of Newell W.Va. produced a range of brightly coloured earthenware called Fiesta Ware. The red glaze produced from 1936-1943 contained uranium oxide, which is slightly radiaoactive.
Vaseline Glass

Vaseline Glass is a particular color of yellow-green glass that is made by adding 2% Uranium Dioxide to the ingredients when the glass formula is made. The addition of the Uranium Dioxide makes the glass color yellow-green. It is also strongly fluorescent under UV light.
Glow In The Dark Clock Hands

The classic case of radiation poisoning. Radium was painted on the hands of clocks, so they would glow in the dark. The radium was painted by women, who had the bad habit of licking the brush tips to form them, ingesting the radium.
Radioactive Record Album Dusters

Way back in ancient times, before the compact disc, primitive man listened to recorded music by playing large (up to a foot in diameter) vinyl discs with grooves engraved in them. A diamond tipped needle rested in the groove, and was vibrated by variations in the groove, corresponding to the audio being recorded. Well, enough ancient history... the point is dust would get on the record, and this was a bad thing, as it caused pops and crackles when played back. Rubbing the dust off was a bad idea, it would scratch the vinyl.
Radiation to the rescue! A brush was sold with trace amounts of polonium
in it. This would create a small electrical charge on the brush, which
would attract the dust and safely remove it from the record. These brushes
are still sold. Polonium has a very short half life, so the useful life of
the brush is probably a few years at the most. It appears that the NRC
licensing requires the manufacturer to take back the brush, but I suspect
that many of them end up in the trash.
Tritium Glow Lights

Tritium (heavy hydrogen with two neutrons) is radioactive, emitting beta particles with a low energy of 18 keV, and having a 12 year half life. besides being useful when building hydrogen bombs, it also has numerous other applications.
A variety of items are sold containing tritium and phosphors, which glow in the dark. Examples include compasses, wristwatches, and glow in the dark keychains, as well as emergency exit signs. The tritium betas excite the phosphors, causing them to glow with visible light. A few microns of plastic is sufficient to block the betas, so the items are quite safe. Unless you were to open one, I guess. Some of thse things have several curies of tritium in them. I don't know what the health effects would be of exposure. Probably not as bad as from the phosphors!
Update!
They may not be quite so safe... I decided to place one on a pancake GM detector.
The readings went from 55 CPM background to about 210 CM. I then removed the actual
glass tube (with the tritium and phosphors) from the plastic holder, and the reading
jumped to about 690 CPM. I suspect that the betas are hitting the glass, and creating
x-rays, with a peak energy of 18 keV. The plastic absorbs most, but not all, of them.
Neon Bulbs
I have heard that some neon bulbs had a small amount of a radioactive gas in them, to ionize the gas. This made it easier for the bulb to light. I don't have confirmation of this. I would suspect the gas was Krypton-85.
Thoriated Welding Rods

Thoriated Tungsten Electrodes used in TIG (tungsten inert gas) welding usually contain 2 percent thorium oxide (ThO2 so technically it is thorium dioxide). Thoriated tungsten can typically be identified either by a red colored band for 2 percent or a yellow band for 1 percent ThO2. Thorium was used to help strike and maintain a better arc stability under DC welding vs. AC welding which uses pure tungsten electrodes. A 3/32 inch electrode across a Ludlum 44-9 pancake GM probe will make a nice (and cheap) check source with a reduced likelihood of contamination (as long as you don't weld or grind the electrode!) especially when compared to lantern mantels. It is recommended by the American Welding Society in keeping with ALARA principles to replace the thoriated electrodes with 2% lanthineated (blue color band).
Vacuum Tubes
Radioactive materials were sometimes used as an ionizer in voltage regulators. Tube types 0A2 and 0B2, and 6143. Thorium (thoriated tungsten) in the filament.
Radium Water/Revigator

This was some sort of quack medical fad in the 1920's, much like using magnets, homeopathy, aromatherapy, etc today. The item was a water container, lines with uranium ore. You would pour your water into it, and I guess it would pick up some of the radium, which you would then ingest. This sounds crazy today, but radiation was new back then, and some thought it was a miracle cure.
Thorium Camping Lantern Mantles:
Some camping lantern mantles contain trace amounts of thorium.Why? It involves the property candlelumenescence (sometimes 'thermoluminescence' or just 'incandescence'). Essentially it means something emits light very efficiently when it's in a flame. Note that this doesn't really explain anything. For instance Yttrium Oxide is used as a phosphor (as in a TV set). So some sort of interesting calalyst/bandgap thing might be going on. Whatever it is, it certainly isn't just plain old incandescence because the light is not generated at 5000 degrees C. Current Coleman lanterns use Yttrium oxide instead.
So I went out to my local WalMart, and picked up two packs, one is made by Coleman, the other is a "no-name" brand. The are both made in India (supposedly mantles made in India are more likely to contain thorium). The Coleman package had no warnings about Thorium on the bag, while the other one did mention it contained a radioactive material, although thorium was not specifically mentioned.
Placing the Coleman mantle on the GM tube had no effect, so I can presume that Coleman no longer uses Thorium (at least not in all their products). The other package was a different matter! Just placing the unopened bag near the GM tube caused the readings to shoot up from a background of about 40 CPM, to around 550 CPM! Removing the mantle from the bag caused the readings to shoot up even higher, to around 9000 CPM. They may have been higher, my homebrew counter only goes up to 9999 counts before it wraps around. With the mantle exposed, I suspect that the GM tube was now detecting Alpha particles that previously were stopped by the plastic bag.
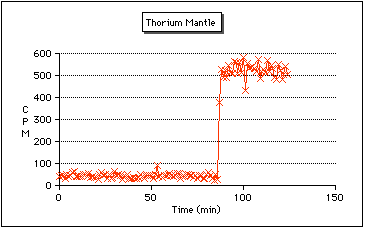
The above graph shows how the count rate significantly increased after the mantle (still in the bag) was placed near the GM tube.
Return to Black Cat Systems' Science Page
cps@blackcatsystems.com Chris Smolinski
 You are visitor number
You are visitor number Modified April 4, 2002
